Pancake bonding pulls conjugated pi-radicals together to short maximum overlap configurations
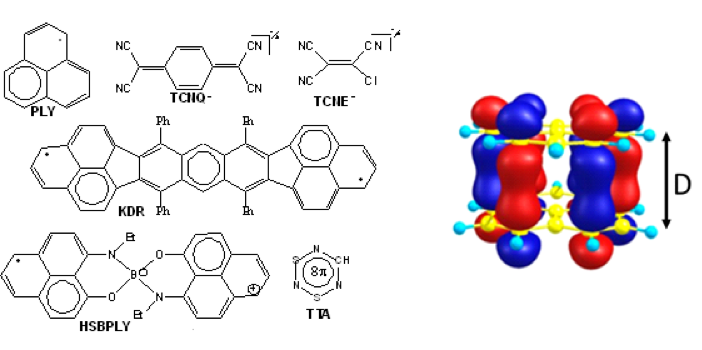
How do molecules stick together to form electronic conducting pathways? One mechanism for generating parallel pi-stacking geometry with maximum orbital overlap is obtained in a form of multicenter electron sharing bonding is called “pancake bonding”. This form of intermolecular interaction is stronger than van der Walls interaction with contact distances (D) shorter than the van der Waals contact. A review of this unique intermolecular interaction is provided in Kertesz, M., 2019. Pancake Bonding: An Unusual Pi‐Stacking Interaction. Chemistry–A European Journal, 25(2), pp.400-416.
The electron sharing is described by a bonding molecular orbital shown in the diagram below on the right, together with a few molecules that display pancake interaction in the solid state.

