Recent Research from Prof. Weiss
Posted in News Story
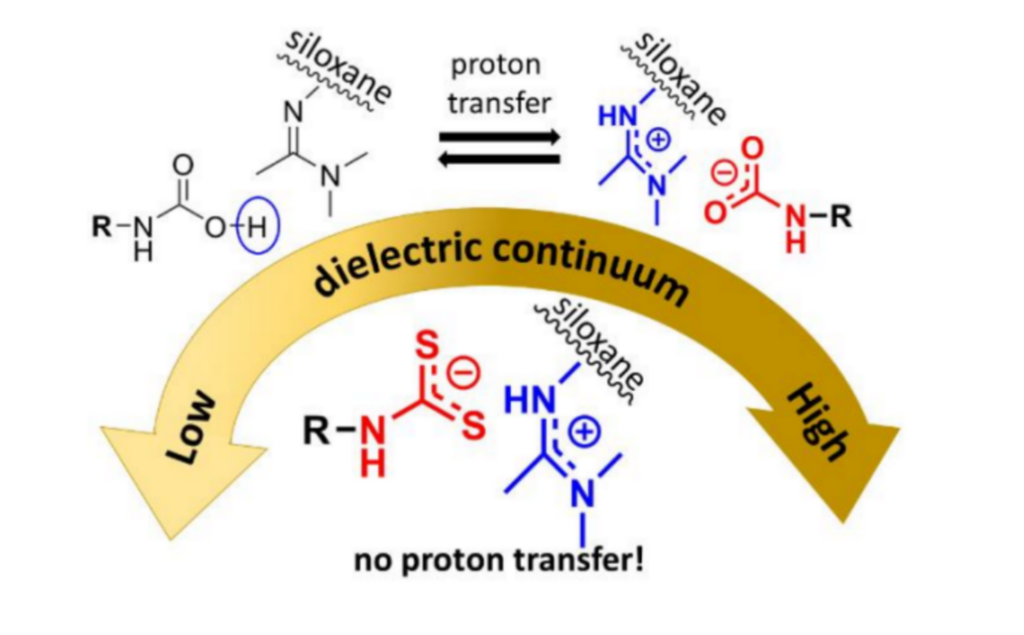
A new publication from Professor Richard Weiss of the ISM, in collaboration with Professor Miklos Kertesz and two Georgetown students, Louis Poon and Jacob Hum, was recently published in the Journal of Physical Organic Chemistry. The research investigates polysiloxane-based ionomors composed of amidinium or imidazolinium groups with alkylcarbamate or alkyldithiocarbamate counterions by using density functional theory (DFT) calculations to explore the dependence of proton transfer on the dielectric constant of the solvent. They found that the ion-pair complexes with counterions in the alkylcarbamic acid form undergo proton transfer in solvents of a specific range of dielectric constant, but no proton transfer occurred in complexes with counterions in alkyldithiocarbamate form. This exploration of proton transfer and its correlation with the principle of proton affinity/pKa equivalence can be useful in further research looking at ion transport within ionic polymers.
