Recent Research from ISMSM Collaboration
Posted in News Story
Two articles authored by members of ISMSM have been accepted in February, 2022 concerning the properties of novel conjugated polymers, both from collaboration between the groups of J. D. Tovar of Johns Hopkins, Ramesh Jasti of University of Oregon (Eugene) and Miklos Kertesz of Georgetown University.
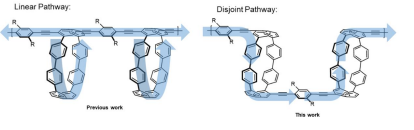
The first, to appear in the Journal of the American Chemical Society, reports the discovery of a novel “disjoint” electronic delocalization pathway along mixed radial and linear conjugated units as proven by a combination of novel synthesis, optical experiments, and quantum mechanical computations. This is illustrated in the figure below indicating a “forced” pathway of delocalization in the “disjoint” design.1 The researchers demonstrated new electronic states from radial/linear mixing in both the small molecules and π-extended polymers.
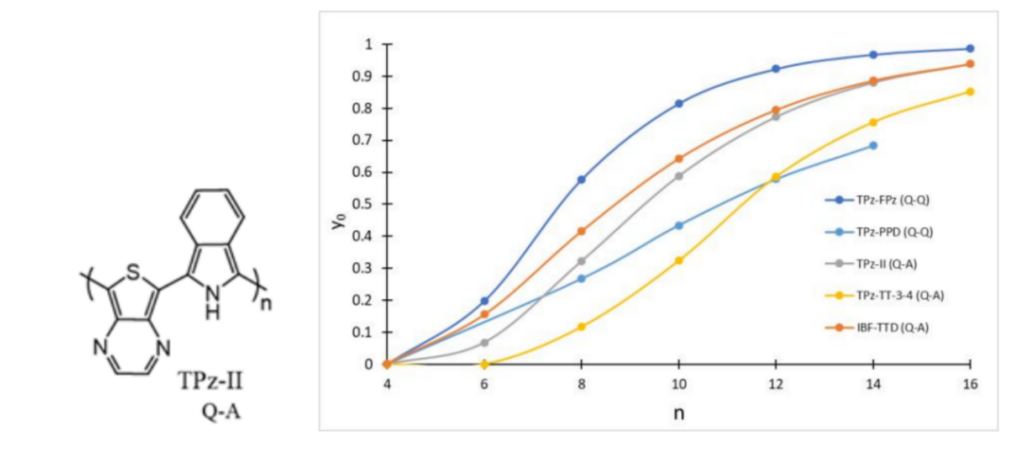
The second work, to appear in the Journal of Physical Chemistry, reports on the dramatic increase of computed diradical character in oligomers of mixed aromatic/quinonoid conjugated units, exemplified for instance by the alternating copolymer illustrated below. So far quinonoid ground state polymers have been rarely identified, and one of the goals of this work was to expand the portfolio of such systems in which over a dozen different repeat units were considered, about half with preferred quinonoid, and half with preferred aromatic structures plus several with the alternating A-Q composition. The system below is one of the combined aromatic-quinonoid copolymers that displays a relatively low bandgap and a high open shell diradicaloid character. The figure below shows how quickly the maximum diradical character is reached as a function of size. Girishma Grover, graduate student in the Department of Chemistry is the first author in this publication.2
This work is being supported by the Basic Energy Sciences program at the US Department of Energy.
1“Splitting the Ring: Impact of Ortho and Meta Pi Conjugation Pathways through Disjointed [8]Cycloparaphenylene Electronic Materials”, Eric Peterson, Ruth L. Maust, Ramesh Jasti, Miklos Kertesz, and John D. Tovar, Journal of the American Chemical Society, https://doi.org/10.1021/jacs.2c00419
2“Quinonoid vs. aromatic π-conjugated oligomers and polymers and their diradical characters”, Girishma Grover, John D. Tovar, and Miklos Kertesz, The Journal of Physical Chemistry, Manuscript ID: jp-2022-00113c.R2
