Molecular Nanohoops Reveal Unexpected Topological Transition
Posted in News Story
A new study led by Dr. Miklos Kertesz and his team at Georgetown University, “Quinonoid radial π-conjugation” published in Chemical Science, uncovers a remarkable electronic transformation in a novel class of molecular nanohoops—compact, ring-shaped systems composed of alternating aromatic and quinonoid units. The research investigates how changing the ratio of these two components affects the nanohoops’ electronic structure, leading to surprising and potentially useful behavior.
These radially π-conjugated macrocycles defy expectations for such zero-dimensional systems. As the number of aromatic segments increases, the frontier molecular orbitals—the HOMO and LUMO—move closer together until they eventually invert. This rare orbital crossing marks a topological transition, a concept typically associated with larger, extended materials like polymers or surfaces.
Visualizing Electronic Reorganization
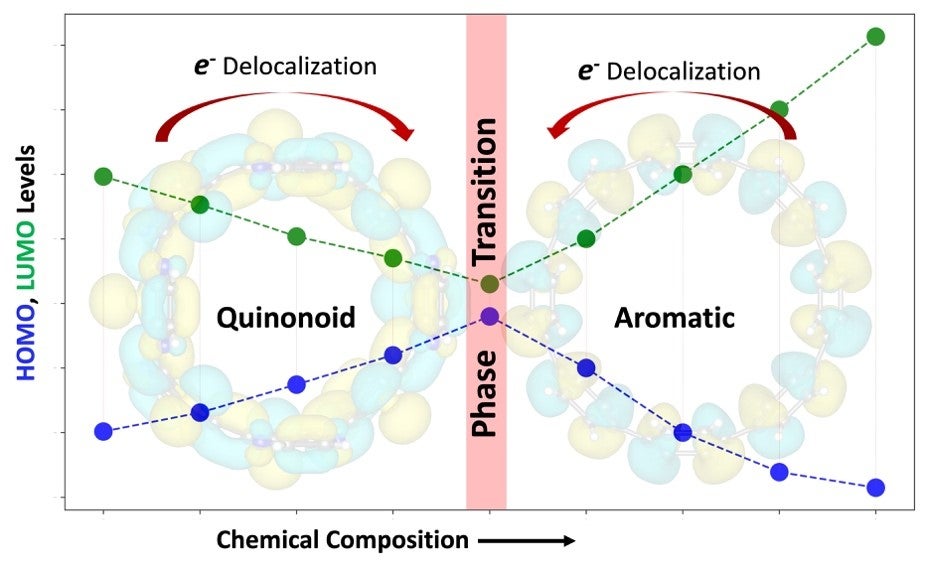
The image captures the striking changes that occur at this critical point in the molecular architecture:
- A dramatic narrowing of the energy gap between the HOMO and LUMO.
- A ground-state spin shift from a singlet (all electrons paired) to a triplet (with two unpaired electrons).
- Precise localization of those unpaired spins at the aromatic–quinonoid junctions.
These electronic and magnetic transitions not only demonstrate the richness of π-conjugation in molecular rings but also suggest that these nanohoops could be engineered to display tunable properties—ideal for applications in molecular spintronics, photophysics, and quantum materials design.
Why It Matters
This work is significant because it shows that even small, closed-loop molecules can host topological transitions previously thought to require extended structures. The ability to trigger an orbital inversion and spin-state change by simply tweaking molecular composition opens new doors in the design of functional organic materials. Such systems could be tailored for responsive behavior, serving as molecular-scale switches, magnetic sensors, or components in future quantum information systems.
About the Researchers
Dr. Rameswar Bhattacharjee is a postdoctoral researcher in Professor Kertesz’s lab.
Dr. John D. Tovar is a Professor in the Department of Chemistry and is also affiliated with the Department of Materials Science and Engineering at Johns Hopkins University.
Dr. Miklos Kertesz is a Professor in Georgetown University’s Department of Chemistry and a member of the Institute for Soft Matter Synthesis and Metrology (ISMSM).
Read the full study in Chemical Science: HERE.